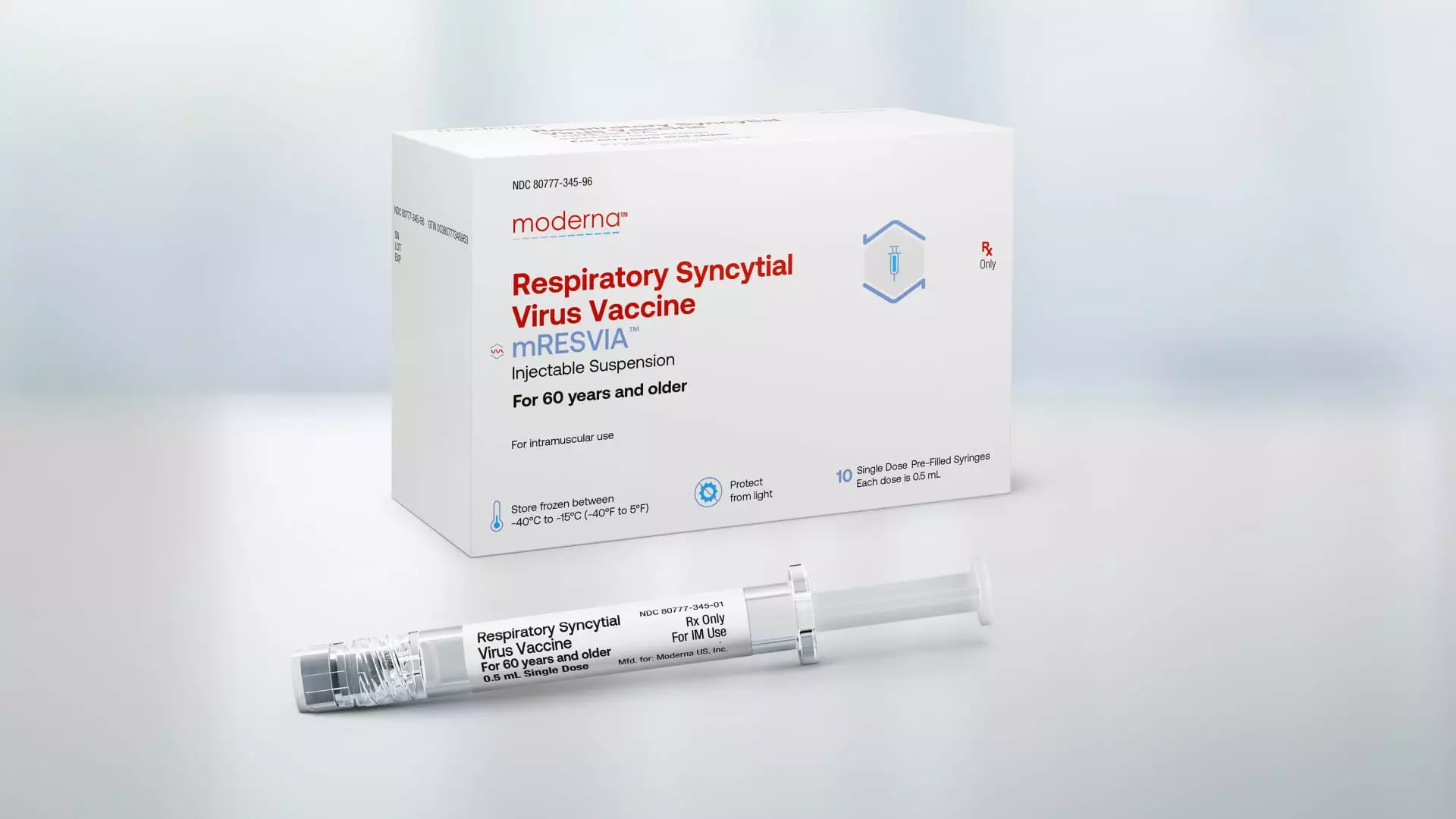
The Food and Drug Administration recently approved Moderna’s vaccine for respiratory syncytial virus (RSV) for adults ages 60 and above. This marks a significant milestone for Moderna as it seeks to diversify its product portfolio beyond its Covid jab. The approval is based on a late-stage trial that demonstrated the vaccine’s efficacy in older adults, who are particularly vulnerable to severe cases of RSV.
Key Features of Moderna’s Vaccine
Moderna’s RSV vaccine, marketed under the brand name mRESVIA, is the first messenger RNA vaccine to receive approval for a disease other than Covid. What sets it apart from existing RSV vaccines is that it is available in a pre-filled syringe, making it easier to administer to patients. The company is expecting a positive recommendation from an advisory panel to the CDC, positioning it to compete with established players like GSK and Pfizer in the RSV vaccine market.
Expanding the Applications of mRNA Technology
Moderna’s success in gaining approval for its RSV vaccine underscores the versatility of its messenger RNA platform. The company is actively pursuing the development of treatments for a range of diseases beyond Covid, including cancer and norovirus. With more than 40 products in its development pipeline, Moderna is poised to make a significant impact in the field of biotechnology.
Investors have shown strong interest in Moderna’s mRNA product pipeline, with the company’s shares rising substantially in recent months. The approval of its RSV vaccine is seen as a positive endorsement of the company’s technological capabilities and growth potential. Moderna’s full-year sales guidance for 2024, which includes revenue from its RSV vaccine, points to a promising future for the company.
Looking ahead, Moderna is focused on advancing its combination shot targeting Covid and the flu, as well as standalone flu vaccines and personalized cancer therapies. The company aims to achieve sales growth by 2025 and break even by 2026 with the launch of new products. By leveraging its expertise in mRNA technology, Moderna is well-positioned to address key public health challenges and drive innovation in the biopharmaceutical industry.
The FDA’s approval of Moderna’s vaccine for RSV represents a significant breakthrough in the fight against a deadly virus that disproportionately impacts older adults. With its cutting-edge mRNA technology and robust product pipeline, Moderna is poised to make a lasting impact on global public health and drive innovation in the field of biotechnology.